Clinical Development
Cancer is complex and requires a multi-faceted treatment approach
To effectively treat cancer, oncologists now recognize that targeting tumor mutations is only part of the the story. The patient’s immune system and underlying medical conditions, such as obesity or systemic metabolic dysfunction, have profound impacts on treatment outcomes.
We’re taking that understanding one step further by leveraging decades of accumulated understanding of the role metabolic hormones (insulin, leptin, adiponectin) have in stimulating oncogenic pathways.
Our all-star team of clinical oncologists, academic researchers, professors and drug development professionals is driven to clinically advance our lead drug evexomostat to treat the whole patient, enhance patients’ lives and offer superior treatment options for physicians.
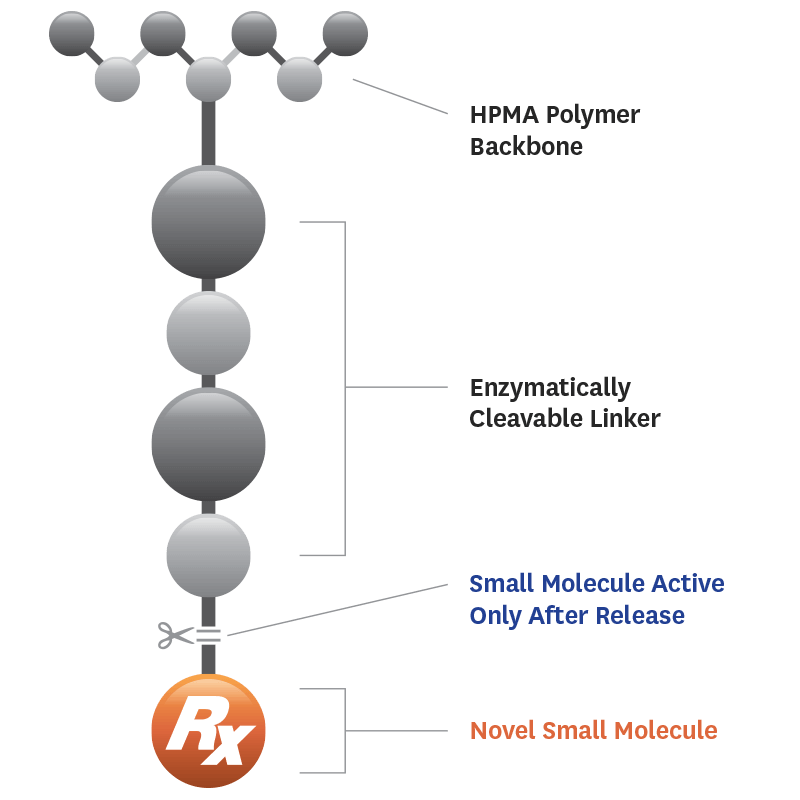
Lead Compound: Evexomostat (SDX-7320)
Our lead compound evexomostat — the first novel therapy that treats both cancer and systemic metabolic hormone dysfunction — is being developed specifically for patients with tumors sensitive to the dysregulated metabolic hormones insulin, leptin and adiponectin.
Learn about what makes evexomostat (SDX-7320) a novel and exciting additional option for patients and clinicians, and our planned and active clinical programs.
-
Pipeline
Review our pipeline of drug candidates under development. -
Clinical Trials
Get the details on SynDevRx-sponsored clinical trials.
SynDevRx Expanded Access Policy
SynDevRx is a clinical-stage biotechnology company focused on developing treatments that address a serious and unmet medical need: cancers that are sensitive to metabolic hormones. It is well established that obesity and diabetes increase the risks for developing cancer, but recent research now shows that these same risk factors also promote cancer and increase its lethality. Our approach combines standard of care therapies with our lead drug evexomostat (SDX-7320) to improve the tumor micro-environment by correcting the systemic metabolic hormones and key angiogenic factors that promote cancer progression and metastasis. We are among the first companies to target this significant yet underfunded area of drug development – an area called ‘metabo-oncology’.
SynDevRx has completed a Phase 1 study and is opening a Phase 1b/2 clinical study in post-menopausal HR+, Her2- metastatic breast cancer patients that have the PIK3CA mutation in combination with standard of care treatment (Piqray, fulvestrant) that are at risk for hyperglycemia. Patients at risk for hyperglycemia with Piqray typically have baseline metabolic dysfunction, such as an HbA1c >5.6% or fasting glucose >100 mg/dL. Enrollment is expected to open in mid 2022 in the US.
Our clinical trials are managed by a team of medical experts and are designed to determine whether the investigational product is safe and effective. These clinical trials will provide insight to the mechanism of action, safety and efficacy of our drug so that the benefits and risks can be adequately quantified, and to determine whether quality of life improvements can be considered against any potential adverse effects. The investigational new drug (IND) approval process ensures treatments have undergone rigorous clinical evaluation to determine whether the benefits outweigh the risks of treatment for the proposed use.
Patients who are interested in participating in one of our trials are encouraged to discuss their specific needs with their oncologist/physician. Information regarding our ongoing clinical trials can be accessed at www.clinicaltrials.gov.
In addition to our clinical trials, SynDevRx has an Expanded Access policy for evexomostat SDX-7320 for eligible patients in the U.S. Expanded Access refers to the use of an investigational drug by an individual patient outside of a clinical trial to prevent or treat a serious condition when the patient is unable to obtain the investigational drug under an IND (e.g., when a patient is not eligible for a clinical study or a clinical study is not recruiting or has been completed).
To qualify for the SynDevRx Expanded Access Program, the patient’s physician must determine that probable risk to the patient from the investigational drug is not greater than the probable risk from the disease or condition. Expanded access is different from a clinical trial where the primary purpose is to collect extensive safety and efficacy data to support submission of an application to the U.S. Food and Drug Administration (FDA) to market a drug.
Physicians seeking additional information regarding Expanded Access for evexomostat (SDX-7320) should submit a request via expandedaccess@syndevrx.com (telephone number +1-617-401-3110). SynDevRx considers providing access to our investigational drug evexomostat (SDX-7320) on a case-by-case basis, dependent on available drug supplies, the physician’s determination as to whether the potential benefit to the patient justifies the potential risks, and company resources. A requesting oncologist/physician should expect to receive a response acknowledging receipt of a request within 5 business days after a completed request has been received. The request for access to a SynDevRx investigational medicine can only be considered if the patient’s treating physician is committed to, and supportive of, the requested treatment.
In accordance with the 21st Century Cures Act, this policy may be revised at any time. Should you have additional questions, please reach out to your oncologist/physician.
