Lead Compound: Evexomostat (SDX-7320)
SynDevRx’s lead compound, evexomostat (SDX-7320), is the first therapy being developed specifically to treat cancer patients who are also overweight or have systemic metabolic dysfunction.
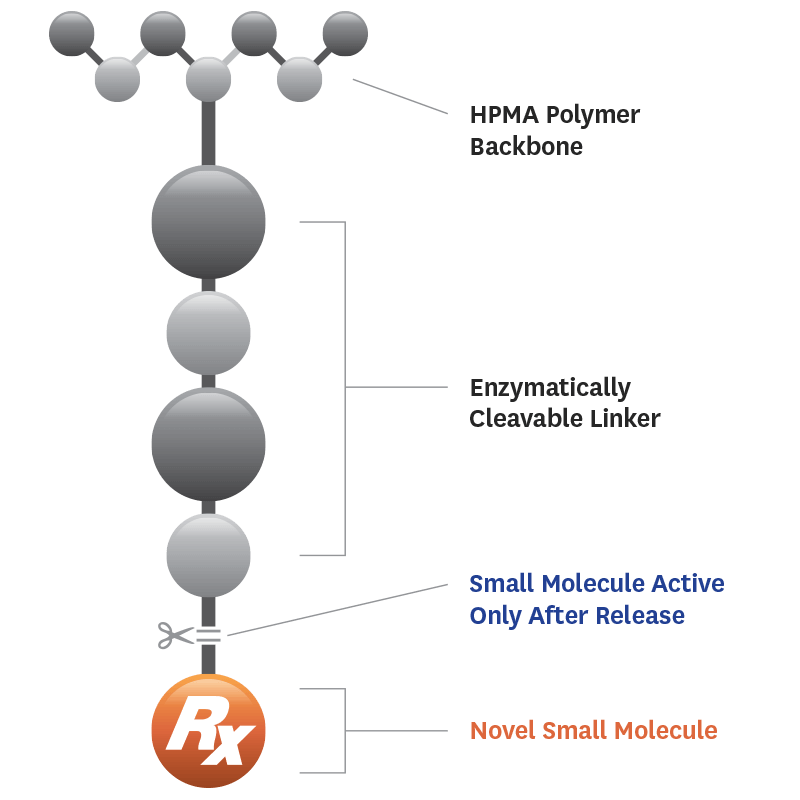
Evexomostat (SDX-7320) is a polymer-drug conjugate that releases a pharmacologically active, novel small-molecule fumagillol derivative in vivo via enzymatic activity.
Fumagillin-class drugs inhibit the enzyme methionine aminopeptidase type II (MetAP2, or p67), which plays an important role in many types of cancer and metabolic disorders.
SynDevRx has been granted composition-of-matter and methods-of-use patents (United States, China, EU, Japan, and many other countries around the world) covering evexomostat (SDX-7320) as well as the small-molecule MetAP2 inhibitor released from SDX-7320. Additional patent applications are pending in territories around the world.
Targeting a Neglected Patient Population
We’re developing our lead compound evexomostat (SDX-7320) to address the well-established link between cancer and dysregulated metabolic hormones.
Our initial target patient population — 2nd line metastatic HR+/Her2- breast cancer with the PIK3CA mutation in combination with drugs from the PI3K/Akt inhibitor class and SERDs — is estimated at over 30,000 new patients per year in the United States and EU 4+1.
Phase 1 results show that evexomostat (SDX-7320) can significantly improve insulin sensitivity that have been shown to lead to treatment resistance for drugs from the PI3K inhibitor class.
Many hormones (e.g., leptin, insulin, estrogen) and inflammatory cytokines (e.g., IL6, TNF-α) are known to promote cancer progression. SDX-7320 acts at the physiological level to systemically decrease the levels of these hormones and cytokines while also targeting the tumor microenvironment (angiogenesis).
Evexomostat (SDX-7320) may also stimulate weight loss, which is associated with significant reductions in leptin and insulin, in obese mice (SDX internal data). The corresponding improvements in insulin resistance and in other elements of systemic metabolic dysfunction have been shown to inhibit tumor growth and metastasis in experimental models of breast cancer and melanoma (SDX data).
Furthermore, in preclinical models, evexomostat (SDX-7320) had a superior safety profile and enhanced efficacy at lower doses than other fumagillin-class MetAP2 inhibitors and with reduced dosing frequency.
*BYL-719 is the original name for Piqray/alpelisib
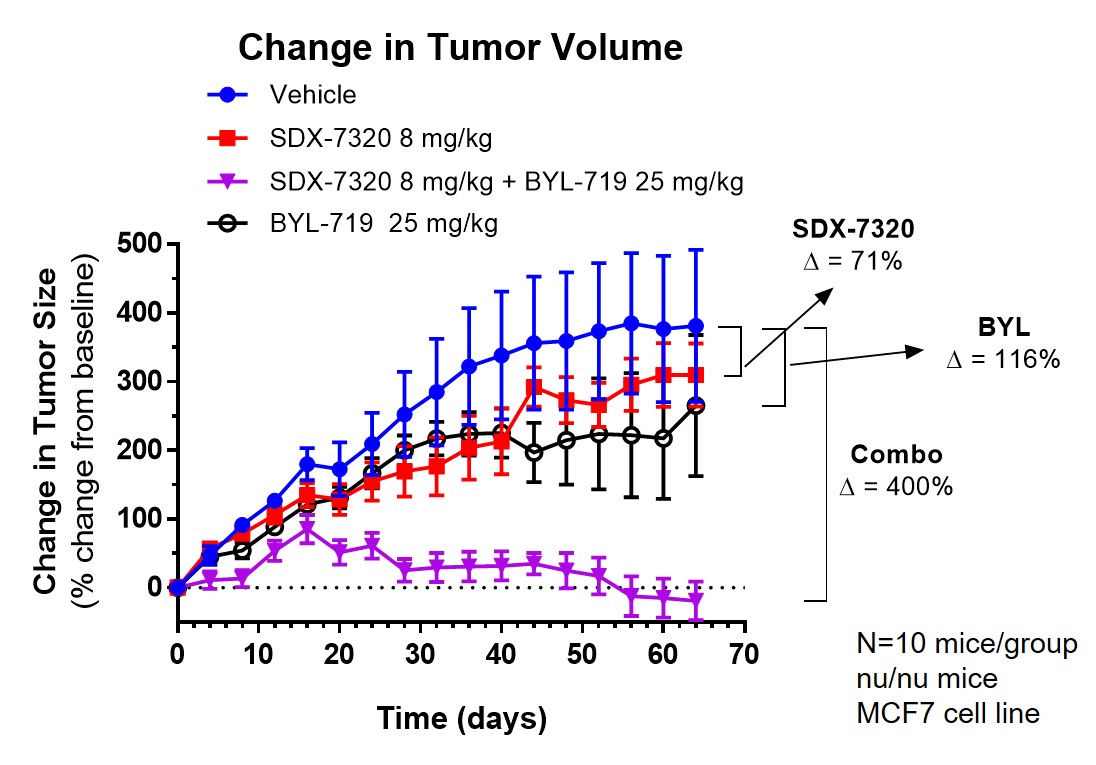
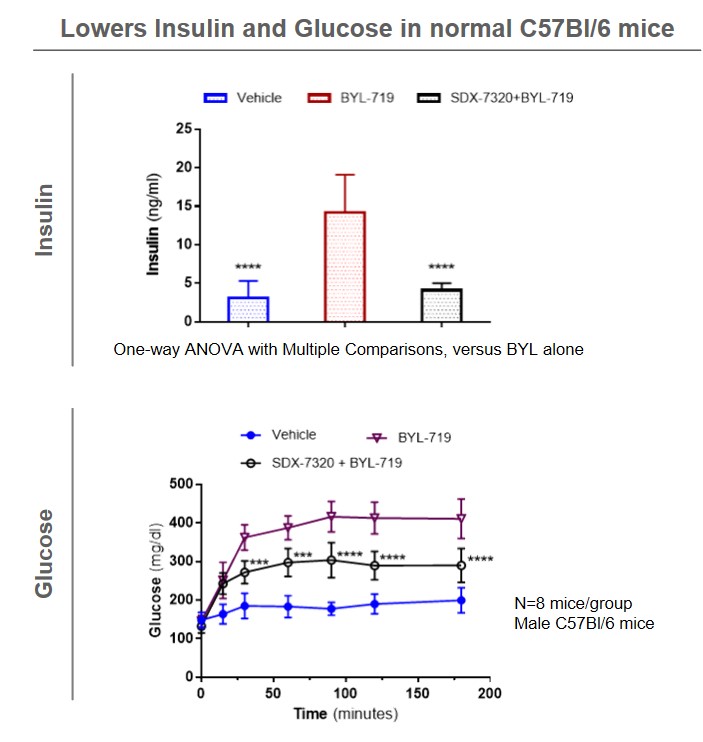
Clinical Programs
SynDevRx is developing evexomostat (SDX-7320) initially for the treatment of postmenopausal women with HR+,Her2-, metastatic breast cancer with the PIK3CA mutation who have progressed following initial therapy with a CKD4/6 inhibitor.
In a recently completed Phase 1 study in late-stage solid tumor cancer patients, evexomostat (SDX-7320) showed significant reductions in fasting insulin and leptin, for cancer patients that had baseline elevated levels (resistance), plus significant increases in the hormone adiponectin.
Additionally, the pro-angiogenic growth factors bFGF (aka FGF2), and VEGF-C were reduced in the Phase 1 study.
The Phase 1 study was a dose escalation safety study in unselected solid tumors. The primary endpoint was to determine the dose and schedule for future clinical studies.
Clinical trials in other indications where metabolic dysfunction is thought to promote the progression of tumors are in the works.
-
Medical and Scientific Advisors
Learn more about the Medical and Scientific Advisory Board Members
