Our Science
Metabo-Oncology
Cutting Off Cancer's Supply Chain
Cancer needs large amounts of glucose, fat and nutrients to grow and spread. Dysregulated metabolic hormones associated with obesity, diabetes and metabolic syndrome help cancer gain access to these energy stores.
Metabo-oncology is the research behind understanding this symbiotic relationship – and disrupting it.
Why it matters
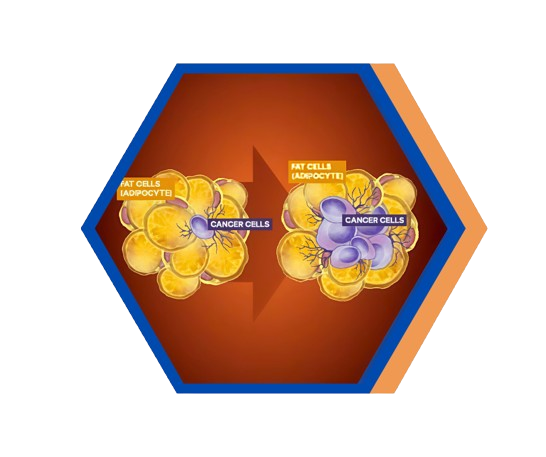
A Needed New Approach to Cancer Treatment
Cancer remains one of the toughest diseases to treat, and dysregulated metabolic hormones like insulin and leptin — often linked to obesity and diabetes — make it even harder.
SynDevRx’s lead compound, Evexomostat (SDX-7320), is a dual-action drug candidate being developed specifically to address this long-neglected unmet medical need.
Evexomostat is intended for cancer patients whose metabolic state may contribute to their disease progression and malignancy.
Baseline Metabolic Dysfunction
Overweight / Obesity
Pre-diabetes
Metabolic syndrome
Dyslipidemia
Type 2 diabetes
Treatment-induced conditions
Hyperglycemia
Hyperinsulinemia
Pre-diabetes
Weight gain
Type 2 diabetes
Highly Vascularized Tumors
Breast cancer
Prostate cancer
Renal cell carcinoma
Hepatocellular Carcinoma (HCC)
Thyroid Cancer
Glioblastoma
Soft tissue cancers (sarcomas)
and Metastatic tumors in general
Please see below for information on clinical trials in progress or planned for SDX-7320.
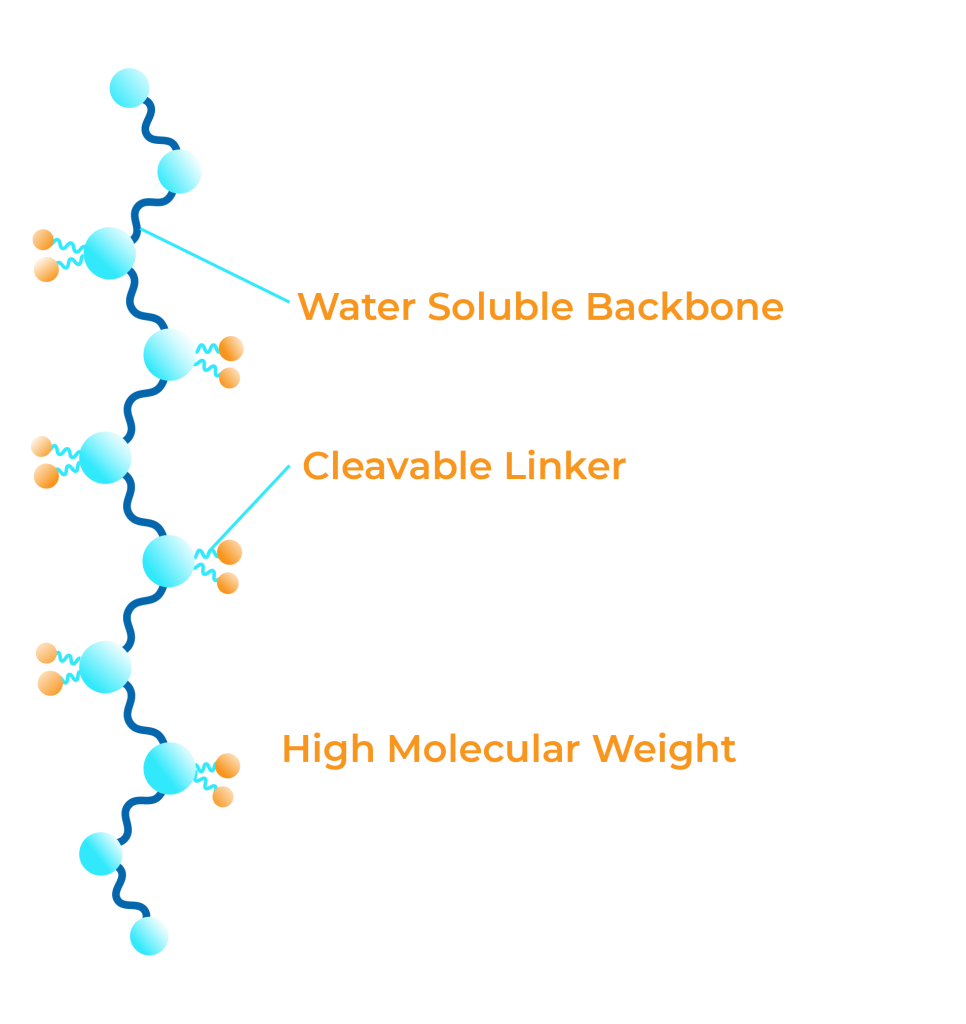
Drug Conjugation
Conjugating, or attaching, small molecules to a polymer backbone, is a clinically validated approach to improving small molecule drug safety and activity.
Optimizing Drug Conjugates
SDX has developed core expertise in improving the safety and efficacy of active small molecules via our deep understanding of polymer-drug conjugation chemistry and manufacturing.
The intrinsic properties of our polymer-drug conjugate change the bio-distribution and pharmacokinetic profile of the active small molecule, making it safer and more effective.
Anti-Angiogenesis, A validated anti-tumor treatment Modality
Cancer demands increasing amounts of oxygen and nutrients to proliferate and metastasize. To achieve this, cancer cells send out signals to the blood vessels, stimulating new blood vessel growth in a complex series of steps called angiogenesis.
Anti-angiogenesis is the treatment modality used to limit a tumor’s access to blood. Our lead drug candidate evexomostat and drugs from the MetAP2 inhibitor class achieve their anti-angiogenic effects by inhibiting endothelial cell proliferation.
What It Is – cutting off the tumor’s access to blood, oxygen and nutrients
Importance – restricting a tumor’s access to blood vessels ‘starves’ the tumor, making combination treatments more effective.
Cancer Indications – Cancers that are highly vascularized and rely on angiogenesis to grow and spread include:
- Breast (TNBC, HR+)
- Prostate
- Glioblastoma
- Renal (clear-cell)
- Hepatocellular
- Thyroid
- Angiosarcoma
Evexomostat is a potent anti-angiogenic drug candidate being developed for use in combination with standard-of-care treatments for metabolically-sensitive cancers.
Scientific Presentations
2025 ESMO
EVADE-1: The methionine aminopeptidase-2 inhibitor, evexomostat, potently suppresses tumor growth in aggressive variant prostate cancer (AVPC) models
SABCS-2024
Amelia-1: Evexomostat Clinical Trials in Progress
AACR-2023
Evexomostat (SDX-7320) Shows Potent Anti-Tumor, Anti-Angiogenic Activity in Multiple Models of Prostate Cancer
SABCS-2022
Evexomostat (SDX-7320) Prepares Amelia-1 Study in Combination with PI3K Inhibitor Plus Fulvestrant
AICR-2022
SDX-7320 Demonstrates Both Anti-Tumor and Anti-Diabetic Activity in Multiple Animal Models
SABCS-2021
SDX-7320 Enhances AKT Inhibitor Activity via Suppression of Hypoxia- and Inflammatory-Related Genes
AACR-2020
SDX-7320 Phase 1 Safety Study Results: Improvements in Angiogenic and Metabolic Markers
SABCS-2019
SDX-7320 Shows Anti-Tumor Synergy and Improves Glucose Control with PI3K Inhibitor
AACR-2019
SDX-7320 Improves Tumor Immune Micro-Environment in TNBC Model (EO771)
AACR-2018
SDX-7320 Enhanced Activity in Models of Obesity-Driven Cancer (EO771 TNBC, B16F10 Melanoma)
AACR-2023
Evexomostat (SDX-7320) Shows Potent Anti-Tumor, Anti-Angiogenic Activity in Multiple Models of Prostate Cancer
AACR-2023
Evexomostat (SDX-7320) Shows Potent Anti-Tumor, Anti-Angiogenic Activity in Multiple Models of Prostate Cancer
SABCS-2022
Evexomostat (SDX-7320) Prepares Amelia-1 Study in Combination with PI3K Inhibitor Plus Fulvestrant
AICR-2022
SDX-7320 Demonstrates Both Anti-Tumor and Anti-Diabetic Activity in Multiple Animal Models
SABCS-2021
SDX-7320 Enhances AKT Inhibitor Activity via Suppression of Hypoxia- and Inflammatory-Related Genes
AACR-2020
SDX-7320 Phase 1 Safety Study Results: Improvements in Angiogenic and Metabolic Markers
SABCS-2019
SDX-7320 Shows Anti-Tumor Synergy and Improves Glucose Control with PI3K Inhibitor
AACR-2019
SDX-7320 Improves Tumor Immune Micro-Environment in TNBC Model (EO771)
AACR-2018
SDX-7320 Enhanced Activity in Models of Obesity-Driven Cancer (EO771 TNBC, B16F10 Melanoma)
1 Broadway Fl. 14, Cambridge, MA 02142
© 2025 Copyright SynDevRx All rights reserved. Privacy Policy | Terms of Use
